Articles
For information related to a specific standard, enter the standard number in the search bar below. If no result is returned, contact your account advisor for guidance.

Be Prepared for Medicare NPE Validation Visits – Part 3: Complaint Procedures
To continue discussing preparation for Medicare National Provider Enrollment (NPE) site visits, let’s turn to the section of the site investigation document dealing with complaints…
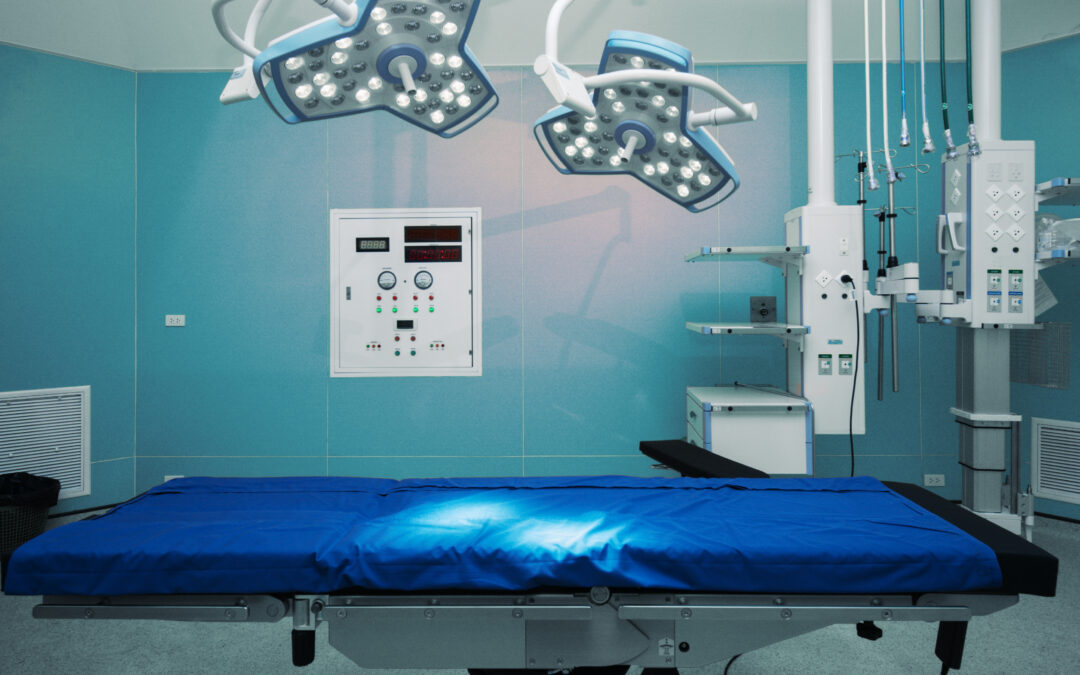
Temperature, Humidity, and Airflow in Your ASC Operating Rooms
Maintaining optimal temperature, humidity, and airflow in your ambulatory surgery center’s operating rooms isn’t just about comfort; it’s a critical measure…

Building an Annual QAPI Plan That Drives Results
In an Ambulatory Surgery Center (ASC), a Quality Assessment and Performance Improvement (QAPI) Program is more than just a regulatory requirement.

Be Prepared for Medicare NPE Validation Visits – Part 2
In August 2024, we wrote about how to prepare for Medicare National Provider Enrollment (NPE) validation or revalidation visits. A continuing uptick in PTAN suspensions and revocations and recent revisions to the site investigation document makes preparing for these inspection visits with thorough planning and attention to detail more important than ever.

Strengthening Pharmaceutical Compliance in ASCs
Safe pharmaceutical practices in ambulatory surgery centers (ASCs) are essential to patient safety, regulatory compliance, and high standards of care. ACHC Standard stipulates that drug preparation and administration align with federal and state laws. This standard is...

Why Your Accreditation End Date Matters
Achieving accreditation is regarded as one of the key benchmarks for measuring the quality of an organization. Once initial accreditation is achieved, it is not permanent and must be maintained and renewed to ensure continued compliance. ACHC Accreditation is valid for a 36-month time period but preparation for renewal should begin at least nine months before the expiration date. Resources are available to help you prepare.